
Fractionating Plain Rasching Rings Columns at Rs 170 /piece Laboratory Glassware ID 4465225988
The mixture is heated at such a rate that the thermometer is at the temperature of the boiling point of the more volatile component. Notice that the thermometer bulb is placed exactly at the outlet from the fractionating column. Relating what happens in the fractionating column to the phase diagram. Suppose you boil a mixture with composition C 1.
The Ultimate Guide to Distillation and Distillation Columns
Step 1 Understanding the concept: A fractionating column is also known as a fractional column. It is an essential piece used in the distillation of liquid mixtures to separate the mixture into its constituent components, or fractions, depending on differences in volatilities.
fractionating column 3d model
No headers. The choice of what fractionating column to use for which application depends in part on availability and the task at hand. Several columns are shown in Figure 5.39: a) Vigreux column with glass indentations, b) Steel wool column made simply be loosely inserting steel wool into the cavity of a fractionating column (similar to a West condenser, but wider), c) Glass beads filled in.
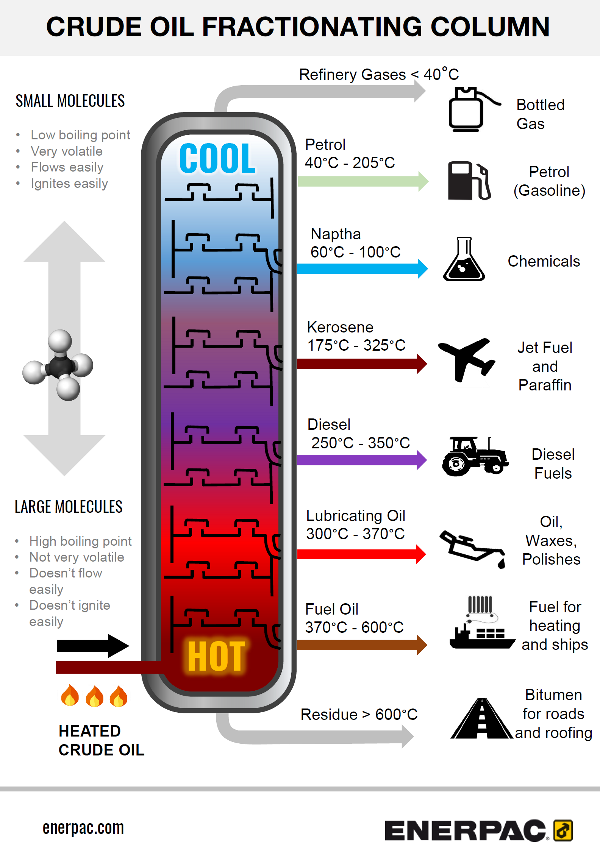
How Crude Oil is Separated Into Fractions Enerpac Blog
A fractionating column essentially allows for many successive distillations to take place at once, without dismantling the apparatus. A fractionating column contains indentations (a Vigreux column, Figure 5.37) or a packing material with lots of surface area. The vapors temporarily condense on these surfaces (see Figure 5.37b) and the heat of.
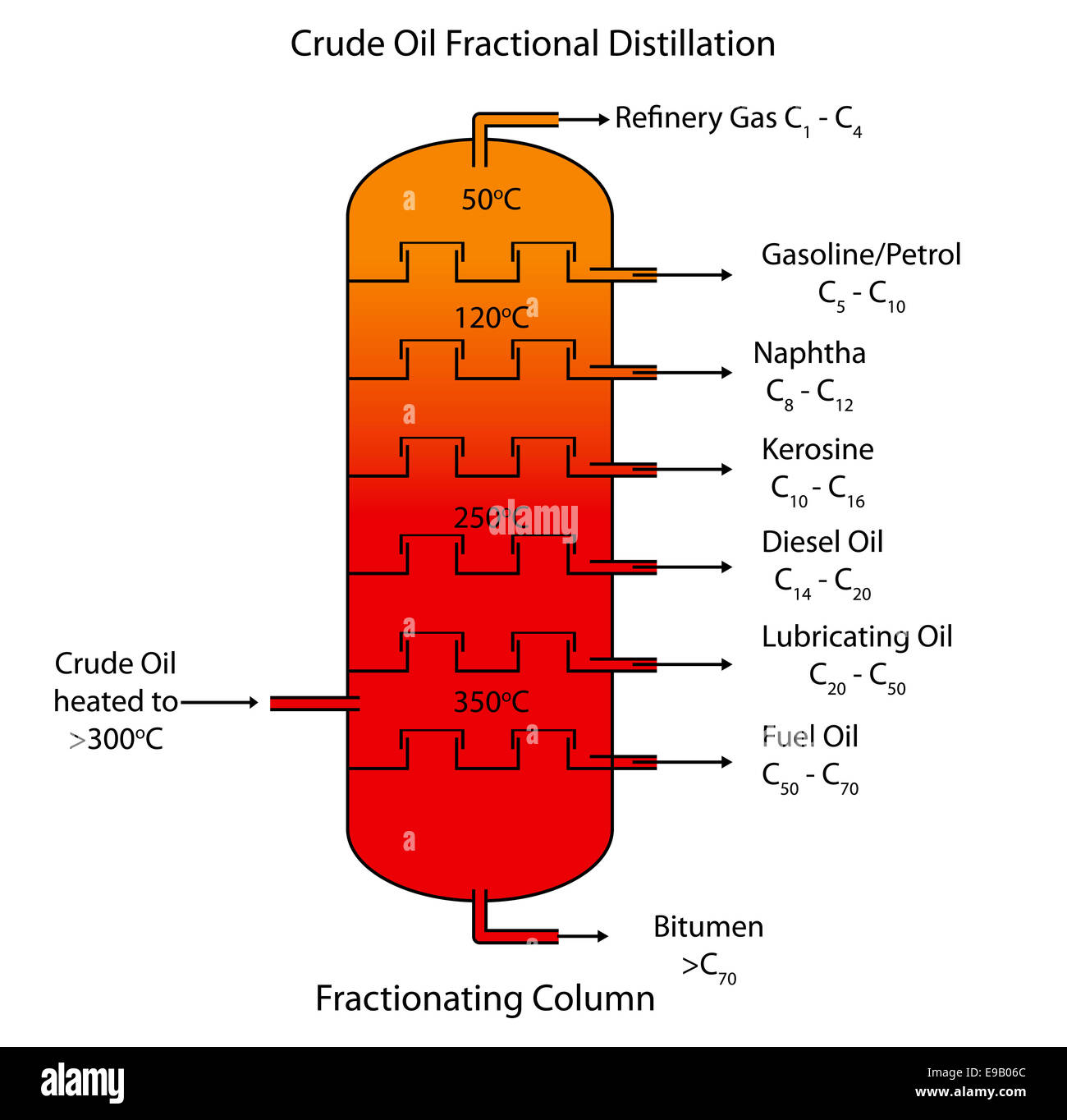
Diagram Showing Fractional Distillation Crude Oil Illustration Stock Riset
Diagram showing the process of fractional distillation to separate crude oil in a fractionating column Fractional distillation is carried out in a fractionating column which is very hot at the bottom and cool at the top Crude oil enters the fractionating column and is heated so vapours rise
Fractionating column
Fractional distillation is a type of distillation which involves the separation of miscible liquids. The process involves repeated distillations and condensations and the mixture is usually separated into component parts. The separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize.

Draw a labelled diagram of the fractionating column.
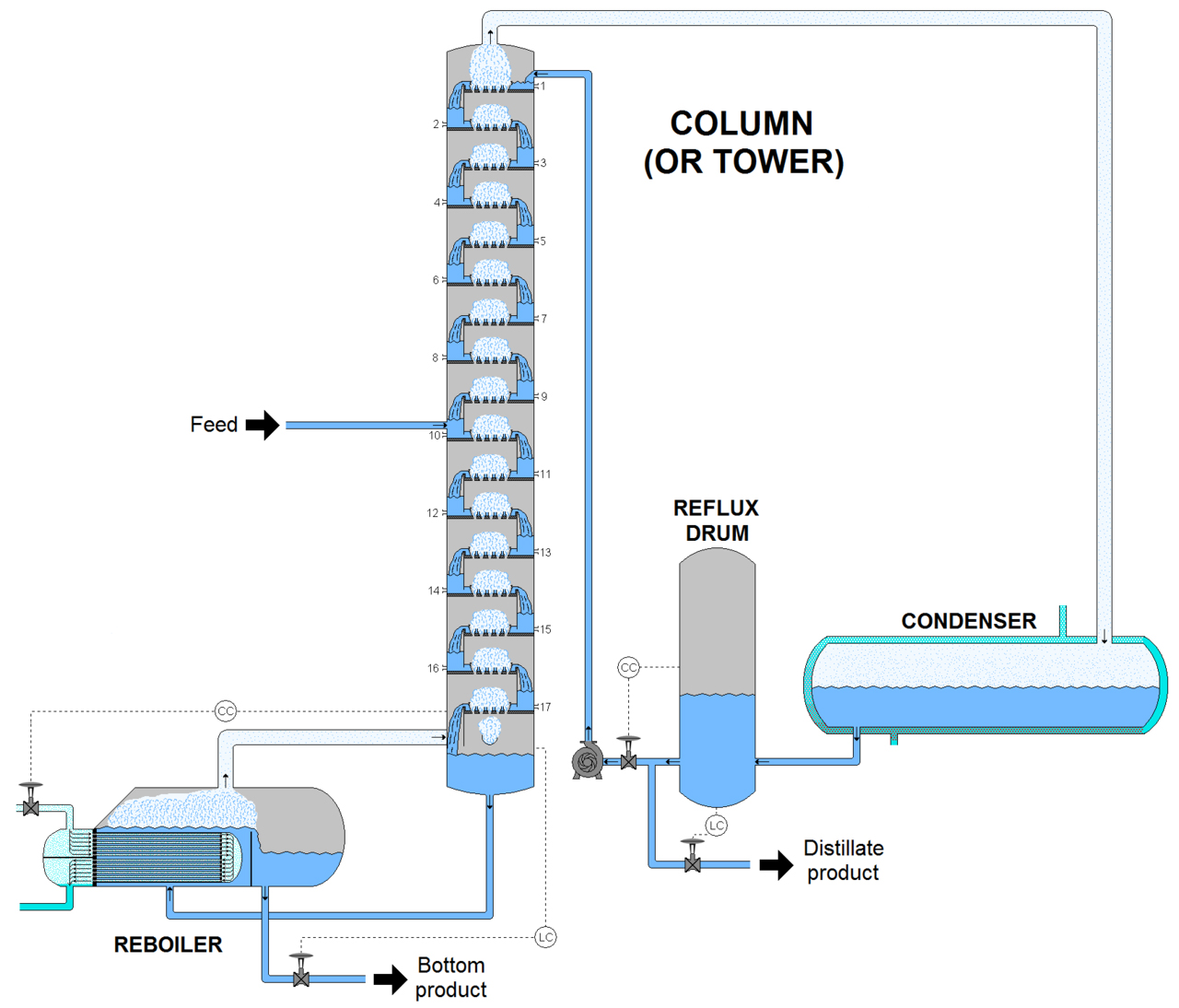
There are two types of fractionating columns: (1) tray column, where trays of various designs are used to hold up the liquid to provide better contact between vapor and liquid, hence better separation; and (2) packed column, where instead of trays, "packings" are used to enhance contact between vapor and liquid phases.

Making Crude Oil Useful—Fractional Distillation and Cracking Owlcation
Figure 1. Diagram of a fractional distillation tower, showing where the different fractions will condense. Note that the temperature is higher at the bottom, so the longer carbon chains will fall out at the bottom, the shorter carbon chains will go up the column until they hit a temperature at which they become liquid.
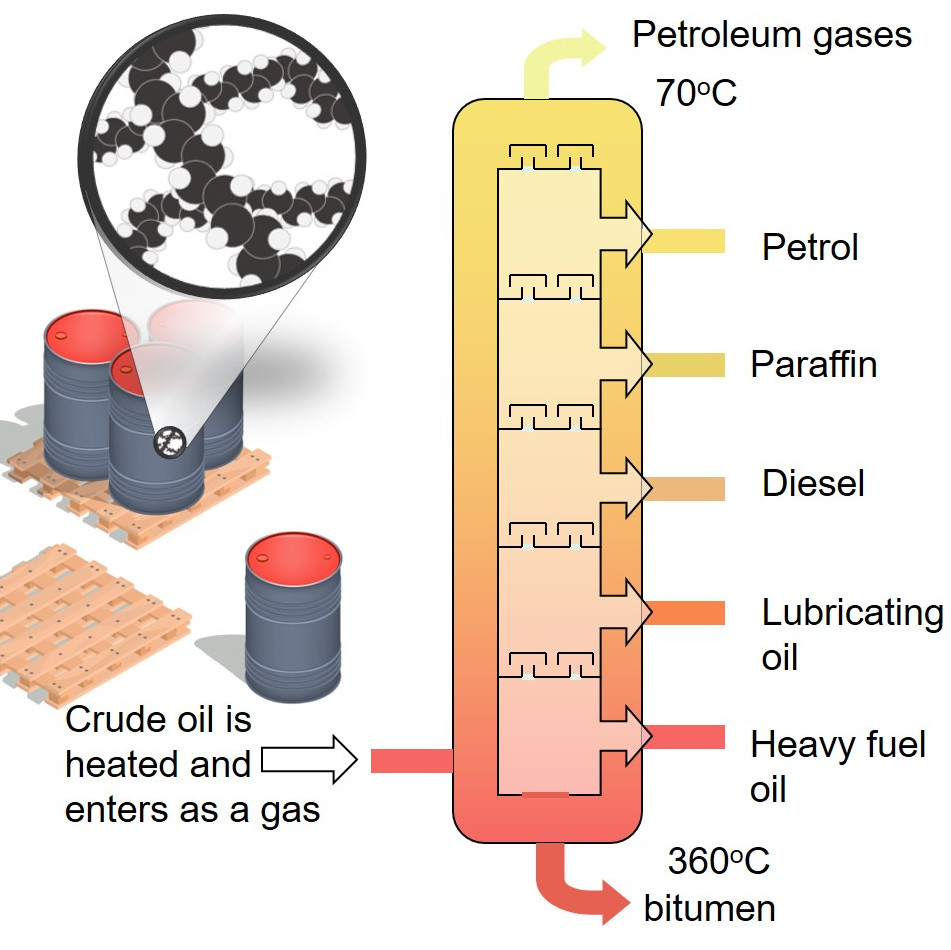
1.10 Fractional distillation
A fractionating column or fractionation column is an essential item used in the distillation of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities.Fractionating columns are used in small-scale laboratory distillations as well as for large-scale industrial distillations.

A fractionating column used in refining (scheme). Download Scientific Diagram
Teacher guidance, including answers to the synoptic question worksheets, as MS Word or pdf. DOWNLOAD ALL. The different hydrocarbons in crude oil must be separated in order to be useful. Fractional distillation separates crude oil into fractions - groups of hydrocarbon molecules with similar carbon chain lengths, properties and boiling points.
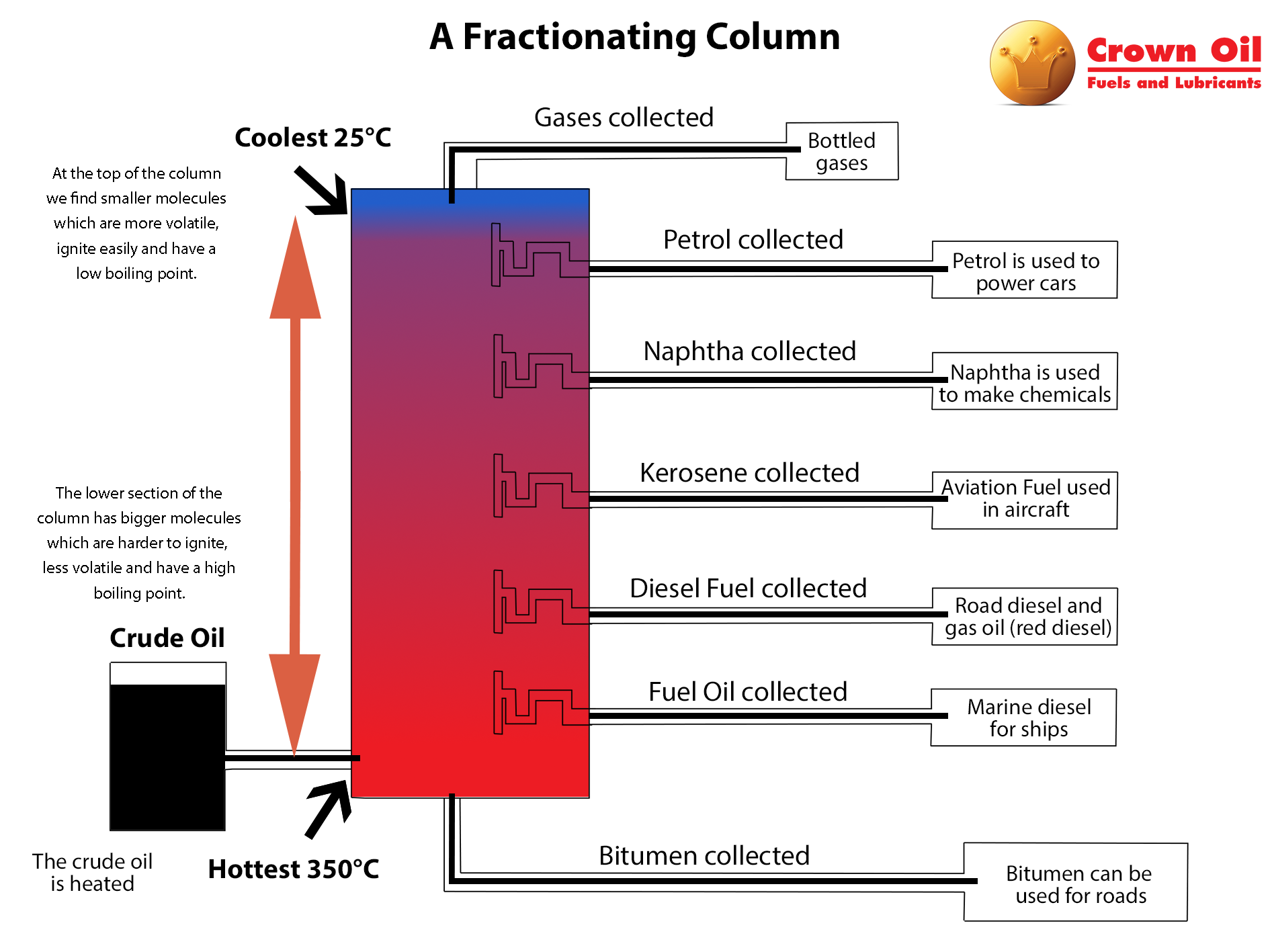
Gas Oil A Reliable Rebated Fuel Crown Oil Blog
This short Tassomai tutorial video explains the basic principles of the fractionating column used to separate the compounds in crude oil.It will help you lea.
fractionating column 3d model
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate.
.jpg)
Fractionation columns are distillation tanks for crude oil
5.3: Fractional Distillation. A simple distillation is incapable of significant purification if the boiling points of the components are too close. When the difference in boiling points is less than 100 ˚C, a modification is necessary, namely insertion of a fractionating column between the distilling flask and three-way adapter.
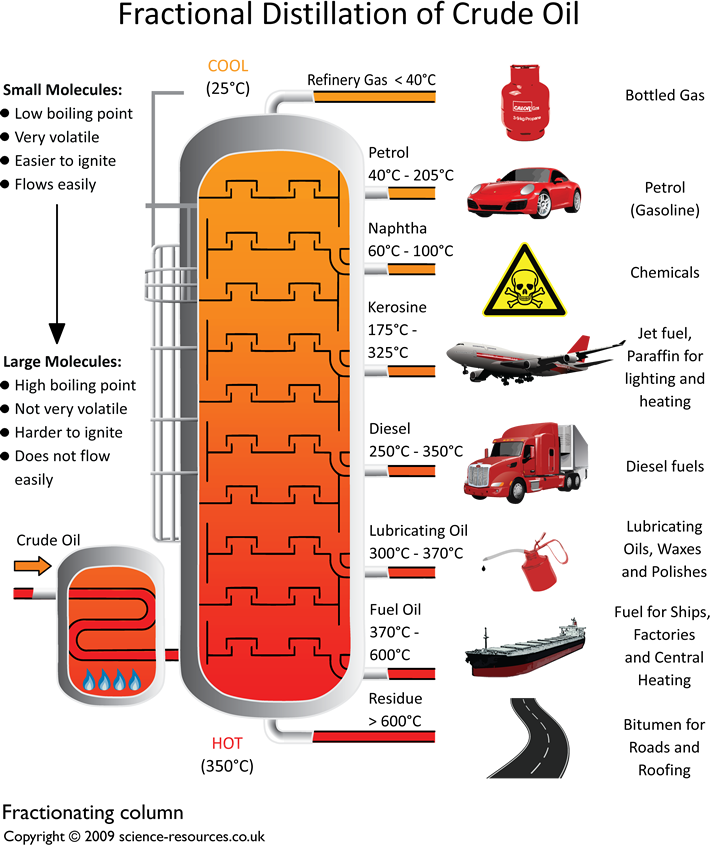
scienceresources.co.uk Fractional distillation of crude oil
The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. Note that the gases leave at the top of the column, the liquids condense in the.

What is Fractional Distillation? The Chemistry Blog
It is assumed that readers have previously performed a simple distillation, so in this section are described differences between simple and fractional distillation. Figure 5.43: Fractional distillation apparatus. Figure 5.44: a) Removal of glass wool plug on a beaded fractionating column, b) Insulating the column with foil, c+d) Condensation on.
Fractionating Column, PNG, 2400x2105px, Fractionating Column, Area, Diagram, Display Resolution
Relating what happens in the fractionating column to the phase diagram. Suppose you boil a mixture with composition C 1.The vapor over the top of the boiling liquid will be richer in the more volatile component, and will have the composition C 2.. That vapor now starts to travel up the fractionating column.
